Page 32
(1) In a reaction, 5.3g of sodium carbonate reacted with 6 g of acetic acid. The products were 2.2 g of carbonate dioxide, 0.9g water and 8.2 g of sodium acetate. Show that these observations are in agreement with the law of conservation of mass.
Sodium carbonate + acetic acid → sodium acetate + carbon dioxide + water
Ans-
Chemical reaction is –
Sodium carbonate + acetic acid → sodium acetate + carbon dioxide + water

 (3) Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
(3) Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Ans- The postulate of Dalton’s atomic theory is the result of the law of conservation of mass is “Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.“
(4) Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Ans- “The relative number and kinds of atoms are constant in a given compound “is the postulate of Dalton’s atomic theory can explain the law of definite proportions.
Page 35
(1) Define the atomic mass unit.
Ans-
Atomic mass unit is used to measure atomic mass of a substance. Its symbol is “amu” used earlier. But after recommendations of the IUPAC, it is written as “u”.
(2) Why is not possible to see an atom with naked eyes?
Ans- Atoms can not exist alone. Atoms are very small particle in size. They form molecules and ions. These molecules and ions combines and form matter that we can feel. Therefore, we can not see an atom with naked eyes.
Page 39
(1) Write down the formulae of
(i) sodium oxide
(ii) aluminium chloride
(iii) sodium suphide
(iv) magnesium hydroxide
Ans-

 (2) Write down the names of compounds represented by the following formulae:
(2) Write down the names of compounds represented by the following formulae:
(i) Al2(SO4)3
(ii) CaCl2
(iii) K2SO4
(iv)KNO3
(v)CaCO3
Ans-
(i) Al2(SO4)3 – Aluminium sulphate
(ii) CaCl2 – Calcium chloride
(iii) K2SO4 – Potassium sulphate
(iv)KNO3 – Potassium nitrate
(v)CaCO3 – Calcium carbonate
(4) How many atoms are present in a
(i) H2S molecule and
(ii) PO43 – ion?
Ans-
(i) 2 atom of hydrogen and one atom of Sulphur, ie, total 3 atoms are present in H2S molecule.
(ii) 1 atom of phosphorus and four atom of Oxygen, ie, total 5 atoms are present in PO43 – ion.
Page 40
(1) Calculate the molecular masses of H2, O2. Cl2, CO2, CH4, C2H6, C2H4, NH3, CH3OH
Ans-



 (2) Calculate the formula unit masses of ZnO, Na2O, K2CO3, given atomic masses of Zn = 65 u, Na = 23 u, K = 39, C = 12 u, and O = 16 u.
(2) Calculate the formula unit masses of ZnO, Na2O, K2CO3, given atomic masses of Zn = 65 u, Na = 23 u, K = 39, C = 12 u, and O = 16 u.
Ans-




 (2) When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
(2) When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
Ans-Mass of the reactant carbon = 3.0 g
Mass of the reactant oxygen = 50.0 g
Total mass of the reactants
= Mass of the reactant carbon + Mass of the reactant oxygen
= 3.0 + 50.0
= 53.0 g
Total mass of the product =Total mass of the reactants
= 53.0 g
Law of conservation of mass will govern our answer.
(3)What are polyatomic ions? Give examples.
Ans- When two or more ions considered as single unit, are called polyatomic ion.
Eg:- Hydroxide ion consists one ion of oxygen and one ion of hydrogen.
(4) Write the chemical formula of the following.
(a) Magnesium chloride
(b) Calcium oxigde
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate
Ans-

 (5) Give the name of the elements present in the following compounds.
(5) Give the name of the elements present in the following compounds.
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate
Ans-
(a) Quick lime –Calcium and oxygen
(b) Hydrogen bromide – Hydrogen and bromine
(c) Baking powder – Sodium, hydrogen, carbon, oxygen
(d) Potassium sulphate – Potassium, sulphur and oxygen
(6) Calculate the molar mass of the following substances.
(a) Ethyne, C2H2
(b) Sulphur molecule, S8
(c)Phosphorus molecule, P4 (Atomic mass of phosphorus = 31)
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO3
Ans-
(a) Ethyne, C2H2
Molecular mass of the C2H2
= 2(atomic mass of the carbon) + 2(atomic mass of the hydrogen)
= 2(12) + 2(1)
=24 + 2
= 26 u
Molar mass = Molecular mass
Molar mass of Ethyne = Molecular mass of Ethyne
= 26 g
Hence, molar mass of the C2H2 is 26 g.
(b) Sulphur molecule, S8

 (7) What is the mass of –
(7) What is the mass of –
(a) 1 mole of nitrogen atoms?
(b) 4 moles of aluminum atoms (Atomic mass of aluminium = 27)?
(c) 10 moles of sodium sulpphite (Na2SO3)?
Ans-

 (8) Convert into mole.
(8) Convert into mole.
(a) 12 g of oxygen gas
(b) 20 g of water
(c) 22 g of carbon dioxide
Ans-


 (9) What is the mass of:
(9) What is the mass of:
(a) 0.2 mole of oxygen atoms?
(b) 0.5 mole of water molecules?
Ans-
 (10) Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
(10) Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
Ans-

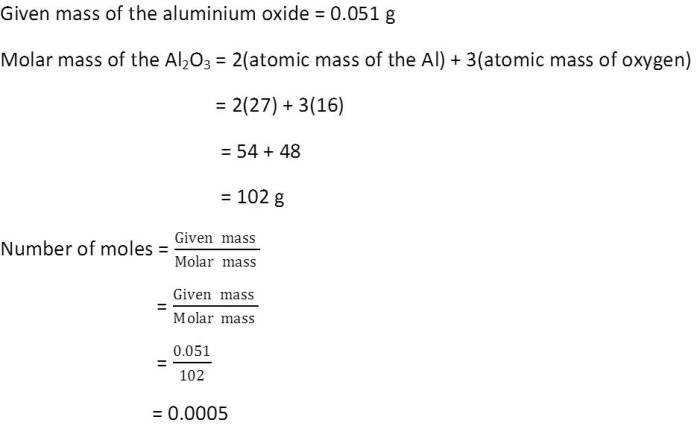
(11) Calculate the number of aluminium ions present in 0.051g of aluminium oxide.
(Hint: The mass of an ion is the same as that of an atom of the same element. Atomic Mass of Al = 27u)
Ans-

Helping Topics
